The U.S. The Food and Drug Administration approved the Cosela drug (trilaciclib) to use as the first therapy to decrease the number of chemotherapy-induced bone marrow suppression in grown-ups.
What Is Cosela and How Is It Used?
Cosela (trilaciclib) is a drug that helps protect bone marrow cells. Trilaciclib is known as the first therapy by inhibiting cyclin-dependent kinase 4/6, which is a type of enzyme. It is the first therapy in its class that was authorized for medicinal use in the USA in February 2021.
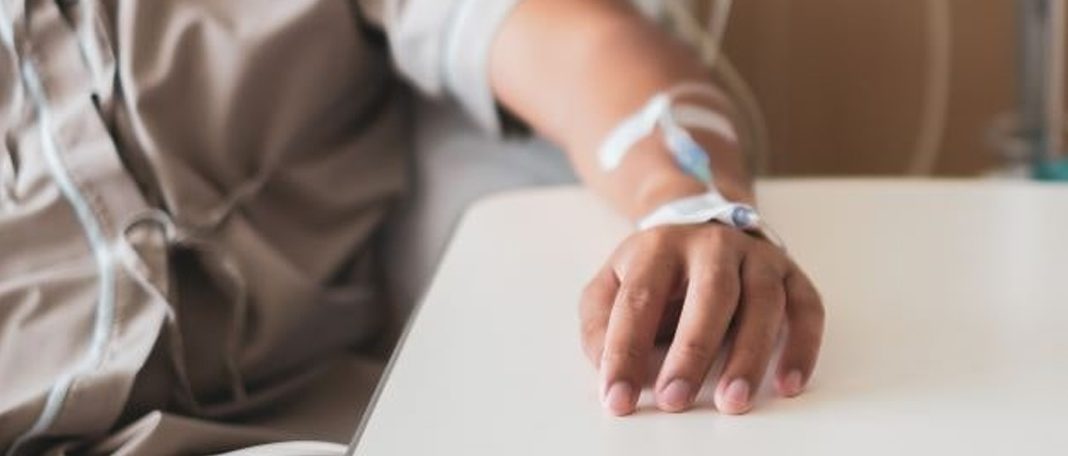
Chemotherapy drugs are produced to kill cancer cells but also can affect normal tissues as well. The bone marrow is especially susceptible to chemotherapy impairment. The bone marrow creates white blood cells, red blood cells, and platelets (tiny fragments in the blood) that transfer fight infection, oxygen, and prevent bleeding. When damaged, the bone marrow makes fewer of these cells that lead to face fatigue, infection, bleeding, and other problems. Trilaciclib may preserve bone marrow cells from the harmful effects of chemotherapy.
The Reason behind FDA Approval on Cosela Drug
The FDA granted authorization to this drug to treat patients with extensive-stage small-cell lung cancer (SCLC).
The Cosela drug may protect bone marrow cells from damage induced by chemotherapy. The authorization was based on outcomes from a combined examination of intent-to-treat datasets from three studies of patients who had extensive-stage small cell lung cancer. In this study, patients underwent standard chemotherapy and received either trilaciclib or placebo.
Albert Deisseroth who is a supervisory medical officer in the Division of Non-Malignant Hematology in the FDA’s Center for Drug Evaluation and Research stated in a press release, “For patients with extensive-stage small-cell lung cancer, protecting bone marrow function may help make their chemotherapy safer and allow them to complete their course of treatment on time and according to plan.” He further added, “Today’s approval of [trilaciclib] will give patients a treatment option that can reduce the occurrence of a common, harmful side effect of chemotherapy.”
Trilaciclib is recognized as a first-in-class FDA-entitled Breakthrough Therapy. It is not only treated SCLC but also being evaluated for tumor types and chemotherapy regimens such as metastatic triple-negative breast cancer and colorectal cancer.
The effectiveness of the Cosela drug was observed in 3 randomized studies in patients who had extensive-stage small cell lung cancer. In these studies, 245 patients participated and received either a placebo before chemotherapy or an infusion of Cosela in their veins.
These researches compared the 2 groups for the proportion of patients who had severe neutropenia (a very low number of white blood cells known as neutrophils) and the period of severe neutropenia in the first round of chemotherapy.
In these 3 studies, patients who took Cosela had less chance of facing severe neutropenia compared to the group who received a placebo.
Among those who took neutropenia, patients who took Cosela on average had it for a shorter time than patients who took a placebo.
Low levels of calcium, phosphate, and potassium; fatigue, headache, increased levels of an enzyme called aspartate aminotransferase, and infection in the lungs( also called pneumonia) are the most common side effects of the Cosela drug.
Patients should be informed about the injection’s reactions, interstitial lung disease/pneumonitis (lung tissue inflammation), acute drug hypersensitivity, and embryo-fetal toxicity.
FDA designated Cosela as Priority Review and Breakthrough Therapy. Now FDA approved Cosela to G1 Therapeutics, Inc.