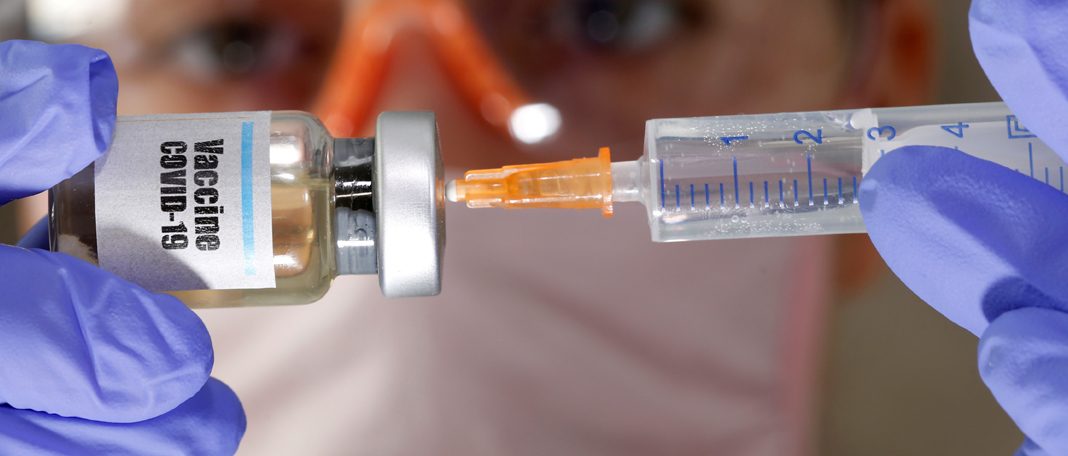
Nearly 11.4 million COVID-19 cases aroused in the United States and 1.3 million people around the world have died due to COVID-19. Which made Pfizer company to bring up the promising development of a vaccine for COVID-19. We have been kept on hearing about the preliminary clinical Pfizer vaccine trials on healthy volunteers. And the final analysis from the last stage of the Pfizer vaccine trials tells that it is 90% effective and it can prevent you against COVID-19.
How Does the Pfizer Vaccine Work?
Pfizer’s vaccine uses a different experimental approach known as messenger RNA, or mRNA, to bring out the immune response in people who receive the vaccine. This method involves injecting a virus genetic code into the body of the volunteers. This promotes the immune system to activate T cells and produce antibodies to destroy infected cells and fight against coronavirus.
How Effective Is Pfizer Vaccine?
Pfizer in collaboration with the German biotech company BioNtech developed the vaccine and released the results of the final analysis. In the final analysis out of 170 confirmed cases of COVID-19, 162 people received a placebo, and 8 received a vaccine. There are no serious adverse effects seen on the volunteers during the treatment.
The most common side effects seen on the volunteers were a pain in the injected area, achy muscles & joints, fatigue which is common among 3.8 percent of people after receiving the first and second doses, and headache which is common among 2 percent of people after receiving the second dose. The older and senior citizens were observed with minor side effects.
The volunteers were assigned to receive two doses of vaccine every 28 days apart. The vaccine had a 90 percent effectiveness after seven days of the second dose vaccination. This is higher than the previous dose vaccination says the Pfizer company.
Dr. Sunil Sood, chair of pediatrics and an infectious disease specialist at Northwell Health’s Southside Hospital said that “This shows that most people who developed infection were placebo recipients, and most of those who got the actual vaccine did not get infected.”
The study on the Pfizer vaccine finds that it is effective among senior citizens above the age of 50 by protecting them against COVID-19 and other infectious diseases. Researchers also welcomed the positive results of the vaccine, and they also insisted that a review of the final data is still needed. After completing the full process the company was about to seek an emergency use authorization from the Food and Development Administration(FDA).
Here is good news related to the COVID-19 vaccine. Yes, If Pfizer got the emergency use approval from the Food and Drug Administration(FDA), then Pfizer will start to release the vaccine for COVID-19 by December. And there are more than hundreds of vaccine-like Sputnik-5, Moderna, developed around the world that is at the final stages of testing.