This was considered the “biggest man-made medical disaster ever” as it lead to the death of over 10,000 children and thousands of miscarriages. In this article, we will discuss why thalidomide is considered one of the biggest medical disasters in history.
What Is Thalidomide?
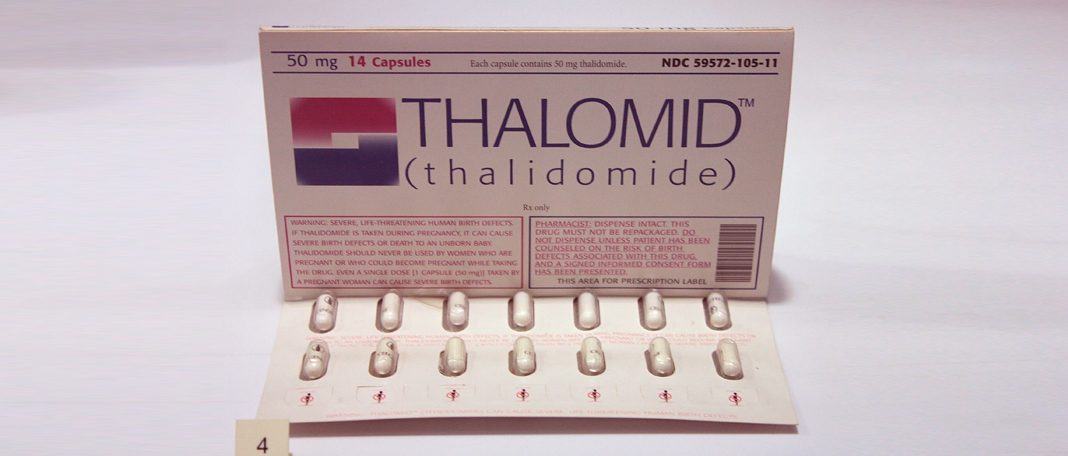
It is a drug that was developed in the 1950s by the West German pharmaceutical company Chemie Grünenthal GmbH. It was intended to be used as sedative or tranquillizer but later was used to treat flu, nausea, and morning sickness in pregnant women. Thalidomide is also used to treat cancers, skin conditions such as leprosy, and HIV associated conditions( Read to find out how HIV is different from AIDS)
History and Impact of the Drug Thalidomide
This drug was deemed to be harmless for humans and was licensed in July 1956 for over-the-counter sale without doctor’s prescription in Germany. Later, the pharmaceutical companies produced and sold the drug under license Chemie Grünenthal GmbH. 14 pharmaceutical companies started marketing thalidomide in 46 countries by the mid-1950s.
In the United Kingdom, it was produced by The Distillers Company(Biochemicals) Ltd, in 1958. They were sold under the brand names Distaval, Tensival, Valgraine, and Asmaval. These drugs were advertised claiming that Distaval can be given to pregnant women and nursing mothers without any adverse effects on mother or child.
But, thalidomide was not approved for marketing and distribution as it was rejected by the Food and Drug Administration. There were no clinical reports to refute reports of patients who experienced nerve damage in the limbs after the long-term use of thalidomide. Therefore, pharmacologist Frances Oldham Kelsey did not accept the request from the distributing companies as they did not provide appropriate evidence.
There was no specific test done that involved pregnant women using thalidomide. Hence there was no strict control over the use of thalidomide in pregnant women. It took five years to discover the connection between the thalidomide consumed by pregnant women and the impact it made on their children. The UK Government had not issued the warning until May 1962.
Over 10,000 babies were estimated to be affected by the drug worldwide in which half have died in the first few months of being born. The babies who survived lived with the effects of the drug including limb, eye, urinary tract, and heart diseases. The severity of the deformities depended on when the drug was taken during the pregnancy. If it was taken on the 20th day of pregnancy, it caused brain damage; Day 21 lead to eye damage; Day 22 caused ear and face damage; Day 24, arms were damaged and if it was taken up to day 28, leg damage was seen. But if it was taken after 42 days of gestation, no damage to the fetus were noted.
Why Scientists Were Slow to Identify the Connection between Pregnant Women Consuming Thalidomide and Its Effects?
The effects of taking this medicine occurred only after 20 to 37 days of consumption. There was no damage to the fetus, if the medicine is taken after 42 days of gestation.
The damage caused was almost similar to certain genetic conditions that affected the limbs.
What Are the Measures Taken after Identifying the Connection between Pregnancy and Thalidomide?
- Only in 1961, the link between thalidomide and its impact was made public in a letter that was published in The Lancet from an Australian doctor, William McBride.
- On 26th November 1961, the drug was formally withdrawn by Chemie Grünenthal and on 2nd December 1961, the UK distributors followed the same.
But it was still available under several different names. Even after a few years, it was available.
- Later, The Thalidomide Society was formed by the parents of the affected children in 1961. Their aim is to provide mutual support and to seek compensation.
- In 1968, Chemie Grünenthal was brought to trial in Germany. The company made arrangements to compensate German victims. Hence, no one was found guilty.
- A campaign led by the Sunday Times in 1972, helped secure the settlement for children affected by thalidomide in the UK.
After this disaster, many countries introduced strict rules for licensing drugs. The drugs that are intended for human use cannot be approved on the basis of animal testing. In the UK, thalidomide can only be prescribed by doctors under strict measures. If someone is prescribed to use thalidomide, he/she must undergo counselling. The FDA initiated the Drug Efficacy Study Implementation to re-segregate drugs that are already available in the market.
Currently, Thalidomide is used to treat leprosy in many countries after WHO ran a clinical trial on the use of thalidomide for leprosy.